



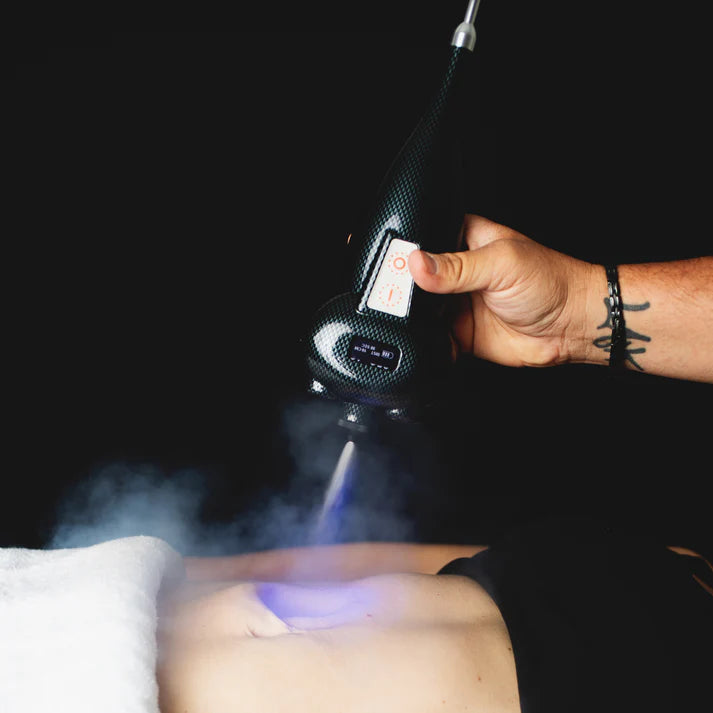


America Cryo Subzero Orthopedic Handheld Cryo Machine
- Description
- ⚕️ CRITICAL HEALTH & SAFETY INFORMATION
- Medical Disclaimer
Subzero Orthopedic Handheld Cryo Machine – Professional Cold Therapy Device for Wellness Applications
The Subzero Orthopedic Handheld Cryo Machine provides targeted cold therapy treatments for wellness and recovery support. Designed for use by trained professionals in clinical, spa, and athletic facility settings, this portable device delivers controlled cooling applications.
Key Applications:
- Recovery Support: May support feelings of comfort after physical activity
- Wellness Treatments: General wellness and self-care applications
- Skin Wellness: May support skin appearance and vitality
- Circulation Support: Cold therapy may promote healthy circulation response
- Professional Settings: Suitable for clinics, spas, wellness centers, athletic facilities
- Targeted Application: Precision delivery to specific body areas
- Portable Design: Handheld format for flexible use
How It Works:
The Subzero system uses pressurized CO₂ gas to deliver rapid, controlled cooling (cryotherapy) to targeted body areas. The sudden temperature change creates a "thermal shock" response that may support the body's natural processes. Cold therapy applications have been used in various wellness and recovery contexts.
Key Features:
✓ Rapid cooling delivery – targeted cold therapy application
✓ Built-in infrared temperature sensors – precision temperature control and monitoring
✓ Distance sensors – helps maintain safe application distance
✓ Rapid-attachment nozzle system – quick setup and easy cleaning
✓ Multiple nozzles included – 5 nozzles + 2 cones for varied applications
✓ Long-lasting battery – cordless operation during treatments
✓ Durable 7' polyurethane-shielded cord – for charging and power
✓ Backlit LCD screen – displays live temperature and treatment data
✓ Pre-set protocols – tailored settings for different body areas
✓ Professional-grade construction – built for clinical/spa use
✓ Portable design – includes protective carrying briefcase
✓ Complete accessory kit – nozzles, cleaning tools, spare parts included
Technical Specifications:
| Specification | Detail |
|---|---|
| Cooling Method | Pressurized CO₂ gas |
| Temperature Control | Built-in infrared sensors with live display |
| Distance Monitoring | Infrared distance sensors |
| Display | Backlit LCD with temperature and treatment data |
| Power | Rechargeable battery with charging cord |
| Cord Length | 7' polyurethane-shielded |
| Nozzle System | Rapid-attachment for quick changes |
| Protocols | Pre-set programs for multiple applications |
| Design | Handheld, portable |
| Professional Use | Designed for trained operators |
What's Included:
Main Components: ✓ Subzero Handheld Cryo Machine
✓ Protective carrying briefcase
✓ Battery charger
Nozzles and Attachments (11 pieces total): ✓ 5 standard nozzles for varied applications
✓ 2 cones for focused treatment
✓ 1 dome attachment
✓ 1 flat massage nozzle
✓ 1 thin contact nozzle
Accessories and Maintenance: ✓ 1 sleeve protection
✓ 1 nozzle cleaning kit
✓ 2 extra O-rings (replacement parts)
✓ 1 tank cover
✓ 2 quick connectors
Shipping:
- Free shipping within the United States
- Professional device requiring trained operator use
Professional Use Requirements:
This device is designed for use by trained professionals in appropriate settings:
- Licensed healthcare facilities
- Professional spa and wellness centers
- Athletic training facilities
- Clinical rehabilitation settings
Operator training required for safe, effective use.
URGENT MEDICAL DISCLAIMER: This cryotherapy equipment is intended for general wellness applications only when operated by trained professionals. Despite marketing language suggesting medical applications, this device:
- Does NOT treat pain, inflammation, or injuries medically
- Does NOT provide medical orthopedic treatment
- Does NOT treat swelling medically
- Does NOT provide medical rehabilitation
- Does NOT reduce fat or cause fat loss ("cryolipolysis" claims are misleading)
- Does NOT stimulate collagen production in any medically significant way
- Does NOT "detoxify" skin tissue (detox claims are pseudoscience)
- Is NOT a medical device for diagnosis, treatment, cure, or prevention of disease
CRITICAL: Medical and Weight Loss Claims are FALSE/MISLEADING
The original marketing makes several problematic claims:
- "Reducing inflammation, pain, and swelling" – These are MEDICAL TREATMENT claims. This device does NOT medically treat these conditions.
- "Orthopedic" and "rehabilitation professionals" – Implies medical treatment applications. This is a wellness device, NOT medical equipment.
-
"Promoting fat reduction through cryolipolysis" – This is a FALSE/MISLEADING claim:
- Medical cryolipolysis (like CoolSculpting) requires FDA-cleared medical devices with specific parameters, controlled cooling, extended application times
- This handheld CO₂ device does NOT perform cryolipolysis
- Cannot cause fat reduction or fat cell destruction
- Completely different technology and application from medical fat reduction procedures
- "Stimulating collagen production" – Not supported by evidence for this type of device
- "Detoxifying skin tissue" – Pseudoscientific claim with no medical basis
Not Evaluated by FDA: Claims about pain relief, inflammation reduction, fat reduction, collagen stimulation, and detoxification have NOT been evaluated by the FDA. This device is NOT FDA cleared or approved for these purposes.
If You Have Pain, Injuries, or Medical Conditions - Seek Proper Medical Care:
Do NOT use cold therapy device as substitute for proper medical treatment:
For Pain/Injuries:
- Consult physician, orthopedist, sports medicine specialist, or physical therapist
- Proper diagnosis essential
- Evidence-based treatments: RICE (Rest, Ice, Compression, Elevation - standard ice is sufficient), physical therapy, medications, injections, surgery when appropriate
- This device does NOT provide medical treatment
For Inflammation:
- Requires proper medical diagnosis and treatment
- This device does NOT medically reduce inflammation
For Fat Reduction:
- This device does NOT cause fat loss
- Evidence-based weight loss: caloric deficit through proper nutrition and exercise
- Medical fat reduction: FDA-cleared procedures (CoolSculpting, etc.) performed by qualified medical professionals
- This handheld device cannot and does not reduce fat
Health Consultation ABSOLUTELY MANDATORY Before Use:
Given the extreme cold hazards and medical claims, consultation with qualified healthcare professional is MANDATORY before use. This device should ONLY be used by trained professionals who understand contraindications.
CRITICAL CONTRAINDICATIONS - DO NOT USE if client/patient has:
Life-Threatening Contraindications:
- Cardiovascular disease or heart conditions: Extreme cold can trigger cardiac events, arrhythmias, heart attack
- Uncontrolled high blood pressure: Cold exposure causes vasoconstriction, dangerous BP spikes
- History of stroke or TIA: Extreme cold increases stroke risk
- Pacemaker or implanted cardiac device: Cold may affect device function
- Severe respiratory conditions: Asthma, COPD—cold can trigger severe bronchospasm or respiratory distress
- Raynaud's disease or phenomenon: Extreme cold causes severe vasospasm, tissue damage risk
- Cold urticaria (cold allergy): Can cause severe allergic reaction, anaphylaxis
- Cryoglobulinemia: Cold causes blood proteins to clump, serious complications
Serious Contraindications:
- Diabetes (especially with neuropathy): Reduced sensation, impaired healing, frostbite risk
- Peripheral neuropathy: Reduced sensation means cannot feel tissue damage occurring
- Peripheral vascular disease: Compromised circulation increases frostbite and tissue damage risk
- Blood clotting disorders or anticoagulant medications: Bleeding risk, impaired healing
- Open wounds, cuts, or skin infections in treatment area
- Recent surgery in treatment area (wait until fully healed)
- Pregnancy or nursing: Unknown effects, not worth any risk
- Cold intolerance or hypersensitivity
- Epilepsy or seizure disorders: Cold stress may trigger seizures
- Thyroid conditions: Cold affects metabolism
- Immune system disorders
- Skin conditions in treatment area: Eczema, psoriasis, dermatitis
- Cancer or history of cancer in treatment area
- Metal implants in treatment area: Metal conducts cold, can cause deep tissue damage
- Children or elderly: Higher risk for adverse effects
Who Should ABSOLUTELY NEVER Use This Device:
❌ Anyone with cardiovascular disease or heart conditions
❌ People with uncontrolled blood pressure
❌ Those with Raynaud's disease or cold sensitivity
❌ Individuals with respiratory conditions (asthma, COPD)
❌ People with diabetes or neuropathy
❌ Those with peripheral vascular disease
❌ Anyone using device for medical treatment of pain, inflammation, or injuries
❌ Anyone expecting fat reduction (device cannot deliver this)
❌ Untrained operators without proper professional training
❌ Settings without proper emergency protocols and equipment
EXTREME SAFETY WARNINGS - LIFE-THREATENING HAZARDS:
- ⚠️ EXTREME COLD HAZARD - FROSTBITE/TISSUE DAMAGE RISK – CO₂ cryotherapy devices deliver EXTREMELY cold temperatures (can reach -78.5°C / -109.3°F at nozzle): Frostbite can occur within SECONDS of direct contact or prolonged close exposure MANDATORY safety protocols:
- Operator MUST be professionally trained in cryotherapy safety
- NEVER apply device in direct contact with skin (maintain proper distance per manufacturer guidelines)
- NEVER keep device stationary over one area—continuous movement required
- Monitor skin response constantly during treatment
- Have emergency frostbite treatment protocol in place
- Know signs of frostbite: numbness, white or grayish-yellow skin, unusually firm or waxy skin, blistering
- STOP immediately if any signs of frostbite or excessive cold injury
- Permanent tissue damage
- Nerve damage
- Amputation of affected tissue in extreme cases
- Scarring and disfigurement
- ⚠️ ASPHYXIATION HAZARD - CO₂ GAS DISPLACEMENT OF OXYGEN – Pressurized CO₂ gas displaces oxygen in enclosed spaces: MANDATORY ventilation requirements:
- Use ONLY in well-ventilated areas with adequate fresh air circulation
- NEVER use in small, enclosed rooms without proper ventilation
- NEVER use in poorly ventilated spaces
- Be aware that CO₂ is heavier than air and accumulates at floor level
- Have CO₂ monitors in treatment areas if possible
- Dizziness, lightheadedness
- Confusion, difficulty concentrating
- Rapid breathing or shortness of breath
- Headache
- Loss of coordination
- Loss of consciousness
- Evacuate area immediately to fresh air
- Seek emergency medical attention
- Do not re-enter until area is properly ventilated
- ⚠️ CARDIAC EVENT RISK - HEART ATTACK/ARRHYTHMIA – Extreme cold exposure causes:
- Vasoconstriction (blood vessel narrowing)
- Increased heart rate and blood pressure
- Stress on cardiovascular system
- Can trigger heart attack, arrhythmias, or stroke in susceptible individuals
- Screen ALL clients for cardiovascular conditions before ANY treatment
- Exclude anyone with heart disease, high blood pressure, stroke history
- Have emergency response plan and equipment (AED) available
- Operator trained in emergency response
- ⚠️ PROFESSIONAL SUPERVISION ABSOLUTELY MANDATORY – This device must ONLY be operated by:
- Trained professionals who have completed proper cryotherapy device training
- In appropriate professional settings (clinics, spas, athletic facilities) with proper safety protocols
- With proper emergency equipment and response capability
- Never for self-treatment or unsupervised use
- Understand all contraindications and screen clients properly
- Know proper application techniques and safety protocols
- Recognize signs of adverse reactions
- Have emergency response training
- Maintain appropriate professional liability insurance
- ⚠️ TREATMENT TIME LIMITS CRITICAL – Excessive cold exposure increases risk:
- Follow manufacturer guidelines for treatment duration on each body area
- Typically 30-90 seconds per area maximum for this type of device
- NEVER exceed recommended exposure times
- Allow skin to return to normal temperature between areas
- Do not treat same area multiple times in one session without proper recovery time
- ⚠️ SKIN PREPARATION AND MONITORING MANDATORY:
- Skin must be completely clean and dry
- Remove all lotions, oils, makeup, products
- Remove jewelry from treatment area
- Visually inspect skin before, during, and after treatment
- Monitor for signs of excessive cold injury
- Stop immediately if skin shows adverse response
- ⚠️ "FAT REDUCTION/CRYOLIPOLYSIS" CLAIM IS COMPLETELY FALSE – This handheld CO₂ device does NOT and CANNOT:
- Reduce fat or destroy fat cells
- Perform cryolipolysis (body contouring)
- Cause weight loss or fat loss
- FDA-cleared medical devices specifically designed for this purpose (e.g., CoolSculpting)
- Controlled, sustained cooling at specific temperatures for 35-60 minutes
- Precise applicators that suction and cool adipose tissue
- Medical oversight and proper patient selection
- NOT a handheld CO₂ spray device with brief application
- ⚠️ "DETOXIFYING" CLAIM IS PSEUDOSCIENCE – Claims about "detoxifying skin tissue" have no scientific basis:
- Human body has liver and kidneys for detoxification
- Cold exposure does not "detox" anything
- This is marketing language without medical meaning
- Do not make detox claims to clients (violates FTC guidelines)
- ⚠️ "ORTHOPEDIC" AND "REHABILITATION" CLAIMS ARE MISLEADING – Device name and marketing suggest medical applications:
- This is NOT medical orthopedic equipment
- Does NOT provide medical rehabilitation
- Should be used for general wellness applications only
- Do not market as medical treatment (regulatory violations)
- ⚠️ OPERATOR LIABILITY AND INSURANCE – Professionals using this device must:
- Carry appropriate professional liability insurance covering cryotherapy
- Understand they assume liability for adverse events
- Have clients sign informed consent forms detailing risks
- Maintain proper records and documentation
- Comply with all local regulations and licensing requirements
- ⚠️ EMERGENCY PREPAREDNESS MANDATORY – Facility using this device must have:
- Emergency action plan for adverse events
- Emergency contact information readily available
- AED (Automated External Defibrillator) on premises
- Staff trained in CPR and emergency response
- First aid supplies including frostbite treatment
- Clear evacuation procedures for oxygen deficiency situations
- ⚠️ DEVICE MAINTENANCE AND SAFETY CHECKS – Regular maintenance critical:
- Inspect device before each use
- Check all connections, seals, O-rings
- Verify temperature sensors functioning properly
- Ensure adequate CO₂ tank pressure
- Replace worn parts promptly
- Follow manufacturer maintenance schedule
- Do not use damaged or malfunctioning equipment
- ⚠️ CO₂ TANK SAFETY – Pressurized gas cylinder hazards:
- Tanks must be properly secured and stored
- Handle according to compressed gas safety protocols
- Do not expose to extreme heat or flame
- Do not drop or damage tanks
- Use only appropriate CO₂ tanks from reputable suppliers
- Follow all compressed gas regulations
Proper Professional Use (If Appropriate):
ONLY use this device if:
- You are a trained professional who has completed proper cryotherapy device training
- You are operating in appropriate professional setting with proper safety protocols, ventilation, and emergency equipment
- You have thoroughly screened client for ALL contraindications
- You have obtained informed consent detailing risks
- You understand this is wellness device, NOT medical treatment equipment
- You have appropriate professional liability insurance
- You will NOT make false claims about fat reduction, medical treatment, or detoxification
If conditions are met: Follow ALL manufacturer instructions and safety protocols meticulously. Screen client thoroughly for contraindications—when in doubt, exclude. Obtain informed consent. Prepare treatment area with proper ventilation. Ensure emergency equipment accessible. Inspect device for proper function. Prepare client skin—clean, dry, no products. Explain treatment to client. Position client appropriately. Follow manufacturer guidelines for distance, movement, and duration for specific body area being treated. Maintain constant movement—NEVER keep device stationary. Monitor skin response continuously. Watch client for any signs of adverse reaction (dizziness, difficulty breathing, excessive cold discomfort, cardiovascular symptoms). Treatment duration typically 30-90 seconds per area maximum—do NOT exceed recommendations. Stop immediately if any adverse signs. Allow skin to recover between areas. Do not treat same area multiple times without proper recovery. After treatment, inspect skin carefully. Provide aftercare instructions. Document treatment. Monitor client for delayed adverse effects. Clean and maintain device properly. Store CO₂ tank safely.
Individual Results Vary:
Cold therapy applications may provide temporary effects such as:
- Temporary skin tightening appearance
- Temporary redness/flushing from circulation response
- Temporary feeling of invigoration
- Relaxation from spa treatment experience
However:
- Effects are temporary and superficial
- No evidence this device provides lasting benefits for claimed applications
- Does NOT reduce fat, stimulate significant collagen, or provide medical benefits
- Results are subjective and largely placebo/expectation-based for wellness applications
Most perceived benefits are likely due to:
- Placebo effect (strong for spa treatments)
- Temporary physiological responses to cold
- Professional attention and care
- Relaxation from treatment experience
Rather than any lasting therapeutic effects.
Realistic Expectations:
What This Device Provides:
- Temporary cold therapy application
- Spa/wellness treatment experience
- Professional service offering for facilities
What This Device Does NOT Provide:
- Medical treatment for pain, inflammation, or injuries
- Fat reduction or body contouring
- Significant collagen stimulation
- "Detoxification" (not a real thing)
- Lasting therapeutic benefits
- Medical rehabilitation
- Anything resembling medical cryolipolysis procedures
Regulatory and Legal Considerations:
Professionals using this device must be aware:
- Making medical claims (pain relief, inflammation reduction, injury treatment) without FDA clearance likely violates regulations
- Making false fat reduction claims violates FTC regulations
- Must comply with all local, state, and federal regulations
- Must have appropriate licensing for services offered
- Must maintain proper liability insurance
- Risk of regulatory action, lawsuits if harm occurs
When to Seek Emergency Medical Care:
Call 911 immediately if client experiences:
- Chest pain or pressure
- Difficulty breathing or severe shortness of breath
- Loss of consciousness or altered mental status
- Severe dizziness or confusion
- Signs of stroke (facial drooping, arm weakness, speech difficulty)
- Severe allergic reaction
- Any life-threatening symptoms
For less severe adverse effects, consult appropriate medical professional.
⚕️ IMPORTANT MEDICAL DISCLAIMER The products sold by Strength & Recovery Solutions are intended for general wellness, fitness, and personal use only. None of our products are medical devices, and they are not intended to diagnose, treat, cure, or prevent any disease, medical condition, or injury. We do not make medical or health claims about any products we sell. Any information provided on this website is for general informational purposes only and does not constitute medical advice. Individual experiences and results vary significantly.
Mandatory Health Consultation: You must consult with a qualified healthcare professional before using any fitness equipment, saunas, cold plunge tubs, cryotherapy devices, red light therapy equipment, or other wellness products—especially if you have pre-existing medical conditions, cardiovascular concerns, are pregnant or nursing, or take medications.
Use at Your Own Risk: Users assume full responsibility for proper installation, use, and safety precautions. Follow all manufacturer instructions. We are not responsible for injuries, damages, or adverse effects resulting from use of our products.

Choose options







- Description
- ⚕️ CRITICAL HEALTH & SAFETY INFORMATION
- Medical Disclaimer
Subzero Orthopedic Handheld Cryo Machine – Professional Cold Therapy Device for Wellness Applications
The Subzero Orthopedic Handheld Cryo Machine provides targeted cold therapy treatments for wellness and recovery support. Designed for use by trained professionals in clinical, spa, and athletic facility settings, this portable device delivers controlled cooling applications.
Key Applications:
- Recovery Support: May support feelings of comfort after physical activity
- Wellness Treatments: General wellness and self-care applications
- Skin Wellness: May support skin appearance and vitality
- Circulation Support: Cold therapy may promote healthy circulation response
- Professional Settings: Suitable for clinics, spas, wellness centers, athletic facilities
- Targeted Application: Precision delivery to specific body areas
- Portable Design: Handheld format for flexible use
How It Works:
The Subzero system uses pressurized CO₂ gas to deliver rapid, controlled cooling (cryotherapy) to targeted body areas. The sudden temperature change creates a "thermal shock" response that may support the body's natural processes. Cold therapy applications have been used in various wellness and recovery contexts.
Key Features:
✓ Rapid cooling delivery – targeted cold therapy application
✓ Built-in infrared temperature sensors – precision temperature control and monitoring
✓ Distance sensors – helps maintain safe application distance
✓ Rapid-attachment nozzle system – quick setup and easy cleaning
✓ Multiple nozzles included – 5 nozzles + 2 cones for varied applications
✓ Long-lasting battery – cordless operation during treatments
✓ Durable 7' polyurethane-shielded cord – for charging and power
✓ Backlit LCD screen – displays live temperature and treatment data
✓ Pre-set protocols – tailored settings for different body areas
✓ Professional-grade construction – built for clinical/spa use
✓ Portable design – includes protective carrying briefcase
✓ Complete accessory kit – nozzles, cleaning tools, spare parts included
Technical Specifications:
| Specification | Detail |
|---|---|
| Cooling Method | Pressurized CO₂ gas |
| Temperature Control | Built-in infrared sensors with live display |
| Distance Monitoring | Infrared distance sensors |
| Display | Backlit LCD with temperature and treatment data |
| Power | Rechargeable battery with charging cord |
| Cord Length | 7' polyurethane-shielded |
| Nozzle System | Rapid-attachment for quick changes |
| Protocols | Pre-set programs for multiple applications |
| Design | Handheld, portable |
| Professional Use | Designed for trained operators |
What's Included:
Main Components: ✓ Subzero Handheld Cryo Machine
✓ Protective carrying briefcase
✓ Battery charger
Nozzles and Attachments (11 pieces total): ✓ 5 standard nozzles for varied applications
✓ 2 cones for focused treatment
✓ 1 dome attachment
✓ 1 flat massage nozzle
✓ 1 thin contact nozzle
Accessories and Maintenance: ✓ 1 sleeve protection
✓ 1 nozzle cleaning kit
✓ 2 extra O-rings (replacement parts)
✓ 1 tank cover
✓ 2 quick connectors
Shipping:
- Free shipping within the United States
- Professional device requiring trained operator use
Professional Use Requirements:
This device is designed for use by trained professionals in appropriate settings:
- Licensed healthcare facilities
- Professional spa and wellness centers
- Athletic training facilities
- Clinical rehabilitation settings
Operator training required for safe, effective use.
URGENT MEDICAL DISCLAIMER: This cryotherapy equipment is intended for general wellness applications only when operated by trained professionals. Despite marketing language suggesting medical applications, this device:
- Does NOT treat pain, inflammation, or injuries medically
- Does NOT provide medical orthopedic treatment
- Does NOT treat swelling medically
- Does NOT provide medical rehabilitation
- Does NOT reduce fat or cause fat loss ("cryolipolysis" claims are misleading)
- Does NOT stimulate collagen production in any medically significant way
- Does NOT "detoxify" skin tissue (detox claims are pseudoscience)
- Is NOT a medical device for diagnosis, treatment, cure, or prevention of disease
CRITICAL: Medical and Weight Loss Claims are FALSE/MISLEADING
The original marketing makes several problematic claims:
- "Reducing inflammation, pain, and swelling" – These are MEDICAL TREATMENT claims. This device does NOT medically treat these conditions.
- "Orthopedic" and "rehabilitation professionals" – Implies medical treatment applications. This is a wellness device, NOT medical equipment.
-
"Promoting fat reduction through cryolipolysis" – This is a FALSE/MISLEADING claim:
- Medical cryolipolysis (like CoolSculpting) requires FDA-cleared medical devices with specific parameters, controlled cooling, extended application times
- This handheld CO₂ device does NOT perform cryolipolysis
- Cannot cause fat reduction or fat cell destruction
- Completely different technology and application from medical fat reduction procedures
- "Stimulating collagen production" – Not supported by evidence for this type of device
- "Detoxifying skin tissue" – Pseudoscientific claim with no medical basis
Not Evaluated by FDA: Claims about pain relief, inflammation reduction, fat reduction, collagen stimulation, and detoxification have NOT been evaluated by the FDA. This device is NOT FDA cleared or approved for these purposes.
If You Have Pain, Injuries, or Medical Conditions - Seek Proper Medical Care:
Do NOT use cold therapy device as substitute for proper medical treatment:
For Pain/Injuries:
- Consult physician, orthopedist, sports medicine specialist, or physical therapist
- Proper diagnosis essential
- Evidence-based treatments: RICE (Rest, Ice, Compression, Elevation - standard ice is sufficient), physical therapy, medications, injections, surgery when appropriate
- This device does NOT provide medical treatment
For Inflammation:
- Requires proper medical diagnosis and treatment
- This device does NOT medically reduce inflammation
For Fat Reduction:
- This device does NOT cause fat loss
- Evidence-based weight loss: caloric deficit through proper nutrition and exercise
- Medical fat reduction: FDA-cleared procedures (CoolSculpting, etc.) performed by qualified medical professionals
- This handheld device cannot and does not reduce fat
Health Consultation ABSOLUTELY MANDATORY Before Use:
Given the extreme cold hazards and medical claims, consultation with qualified healthcare professional is MANDATORY before use. This device should ONLY be used by trained professionals who understand contraindications.
CRITICAL CONTRAINDICATIONS - DO NOT USE if client/patient has:
Life-Threatening Contraindications:
- Cardiovascular disease or heart conditions: Extreme cold can trigger cardiac events, arrhythmias, heart attack
- Uncontrolled high blood pressure: Cold exposure causes vasoconstriction, dangerous BP spikes
- History of stroke or TIA: Extreme cold increases stroke risk
- Pacemaker or implanted cardiac device: Cold may affect device function
- Severe respiratory conditions: Asthma, COPD—cold can trigger severe bronchospasm or respiratory distress
- Raynaud's disease or phenomenon: Extreme cold causes severe vasospasm, tissue damage risk
- Cold urticaria (cold allergy): Can cause severe allergic reaction, anaphylaxis
- Cryoglobulinemia: Cold causes blood proteins to clump, serious complications
Serious Contraindications:
- Diabetes (especially with neuropathy): Reduced sensation, impaired healing, frostbite risk
- Peripheral neuropathy: Reduced sensation means cannot feel tissue damage occurring
- Peripheral vascular disease: Compromised circulation increases frostbite and tissue damage risk
- Blood clotting disorders or anticoagulant medications: Bleeding risk, impaired healing
- Open wounds, cuts, or skin infections in treatment area
- Recent surgery in treatment area (wait until fully healed)
- Pregnancy or nursing: Unknown effects, not worth any risk
- Cold intolerance or hypersensitivity
- Epilepsy or seizure disorders: Cold stress may trigger seizures
- Thyroid conditions: Cold affects metabolism
- Immune system disorders
- Skin conditions in treatment area: Eczema, psoriasis, dermatitis
- Cancer or history of cancer in treatment area
- Metal implants in treatment area: Metal conducts cold, can cause deep tissue damage
- Children or elderly: Higher risk for adverse effects
Who Should ABSOLUTELY NEVER Use This Device:
❌ Anyone with cardiovascular disease or heart conditions
❌ People with uncontrolled blood pressure
❌ Those with Raynaud's disease or cold sensitivity
❌ Individuals with respiratory conditions (asthma, COPD)
❌ People with diabetes or neuropathy
❌ Those with peripheral vascular disease
❌ Anyone using device for medical treatment of pain, inflammation, or injuries
❌ Anyone expecting fat reduction (device cannot deliver this)
❌ Untrained operators without proper professional training
❌ Settings without proper emergency protocols and equipment
EXTREME SAFETY WARNINGS - LIFE-THREATENING HAZARDS:
- ⚠️ EXTREME COLD HAZARD - FROSTBITE/TISSUE DAMAGE RISK – CO₂ cryotherapy devices deliver EXTREMELY cold temperatures (can reach -78.5°C / -109.3°F at nozzle): Frostbite can occur within SECONDS of direct contact or prolonged close exposure MANDATORY safety protocols:
- Operator MUST be professionally trained in cryotherapy safety
- NEVER apply device in direct contact with skin (maintain proper distance per manufacturer guidelines)
- NEVER keep device stationary over one area—continuous movement required
- Monitor skin response constantly during treatment
- Have emergency frostbite treatment protocol in place
- Know signs of frostbite: numbness, white or grayish-yellow skin, unusually firm or waxy skin, blistering
- STOP immediately if any signs of frostbite or excessive cold injury
- Permanent tissue damage
- Nerve damage
- Amputation of affected tissue in extreme cases
- Scarring and disfigurement
- ⚠️ ASPHYXIATION HAZARD - CO₂ GAS DISPLACEMENT OF OXYGEN – Pressurized CO₂ gas displaces oxygen in enclosed spaces: MANDATORY ventilation requirements:
- Use ONLY in well-ventilated areas with adequate fresh air circulation
- NEVER use in small, enclosed rooms without proper ventilation
- NEVER use in poorly ventilated spaces
- Be aware that CO₂ is heavier than air and accumulates at floor level
- Have CO₂ monitors in treatment areas if possible
- Dizziness, lightheadedness
- Confusion, difficulty concentrating
- Rapid breathing or shortness of breath
- Headache
- Loss of coordination
- Loss of consciousness
- Evacuate area immediately to fresh air
- Seek emergency medical attention
- Do not re-enter until area is properly ventilated
- ⚠️ CARDIAC EVENT RISK - HEART ATTACK/ARRHYTHMIA – Extreme cold exposure causes:
- Vasoconstriction (blood vessel narrowing)
- Increased heart rate and blood pressure
- Stress on cardiovascular system
- Can trigger heart attack, arrhythmias, or stroke in susceptible individuals
- Screen ALL clients for cardiovascular conditions before ANY treatment
- Exclude anyone with heart disease, high blood pressure, stroke history
- Have emergency response plan and equipment (AED) available
- Operator trained in emergency response
- ⚠️ PROFESSIONAL SUPERVISION ABSOLUTELY MANDATORY – This device must ONLY be operated by:
- Trained professionals who have completed proper cryotherapy device training
- In appropriate professional settings (clinics, spas, athletic facilities) with proper safety protocols
- With proper emergency equipment and response capability
- Never for self-treatment or unsupervised use
- Understand all contraindications and screen clients properly
- Know proper application techniques and safety protocols
- Recognize signs of adverse reactions
- Have emergency response training
- Maintain appropriate professional liability insurance
- ⚠️ TREATMENT TIME LIMITS CRITICAL – Excessive cold exposure increases risk:
- Follow manufacturer guidelines for treatment duration on each body area
- Typically 30-90 seconds per area maximum for this type of device
- NEVER exceed recommended exposure times
- Allow skin to return to normal temperature between areas
- Do not treat same area multiple times in one session without proper recovery time
- ⚠️ SKIN PREPARATION AND MONITORING MANDATORY:
- Skin must be completely clean and dry
- Remove all lotions, oils, makeup, products
- Remove jewelry from treatment area
- Visually inspect skin before, during, and after treatment
- Monitor for signs of excessive cold injury
- Stop immediately if skin shows adverse response
- ⚠️ "FAT REDUCTION/CRYOLIPOLYSIS" CLAIM IS COMPLETELY FALSE – This handheld CO₂ device does NOT and CANNOT:
- Reduce fat or destroy fat cells
- Perform cryolipolysis (body contouring)
- Cause weight loss or fat loss
- FDA-cleared medical devices specifically designed for this purpose (e.g., CoolSculpting)
- Controlled, sustained cooling at specific temperatures for 35-60 minutes
- Precise applicators that suction and cool adipose tissue
- Medical oversight and proper patient selection
- NOT a handheld CO₂ spray device with brief application
- ⚠️ "DETOXIFYING" CLAIM IS PSEUDOSCIENCE – Claims about "detoxifying skin tissue" have no scientific basis:
- Human body has liver and kidneys for detoxification
- Cold exposure does not "detox" anything
- This is marketing language without medical meaning
- Do not make detox claims to clients (violates FTC guidelines)
- ⚠️ "ORTHOPEDIC" AND "REHABILITATION" CLAIMS ARE MISLEADING – Device name and marketing suggest medical applications:
- This is NOT medical orthopedic equipment
- Does NOT provide medical rehabilitation
- Should be used for general wellness applications only
- Do not market as medical treatment (regulatory violations)
- ⚠️ OPERATOR LIABILITY AND INSURANCE – Professionals using this device must:
- Carry appropriate professional liability insurance covering cryotherapy
- Understand they assume liability for adverse events
- Have clients sign informed consent forms detailing risks
- Maintain proper records and documentation
- Comply with all local regulations and licensing requirements
- ⚠️ EMERGENCY PREPAREDNESS MANDATORY – Facility using this device must have:
- Emergency action plan for adverse events
- Emergency contact information readily available
- AED (Automated External Defibrillator) on premises
- Staff trained in CPR and emergency response
- First aid supplies including frostbite treatment
- Clear evacuation procedures for oxygen deficiency situations
- ⚠️ DEVICE MAINTENANCE AND SAFETY CHECKS – Regular maintenance critical:
- Inspect device before each use
- Check all connections, seals, O-rings
- Verify temperature sensors functioning properly
- Ensure adequate CO₂ tank pressure
- Replace worn parts promptly
- Follow manufacturer maintenance schedule
- Do not use damaged or malfunctioning equipment
- ⚠️ CO₂ TANK SAFETY – Pressurized gas cylinder hazards:
- Tanks must be properly secured and stored
- Handle according to compressed gas safety protocols
- Do not expose to extreme heat or flame
- Do not drop or damage tanks
- Use only appropriate CO₂ tanks from reputable suppliers
- Follow all compressed gas regulations
Proper Professional Use (If Appropriate):
ONLY use this device if:
- You are a trained professional who has completed proper cryotherapy device training
- You are operating in appropriate professional setting with proper safety protocols, ventilation, and emergency equipment
- You have thoroughly screened client for ALL contraindications
- You have obtained informed consent detailing risks
- You understand this is wellness device, NOT medical treatment equipment
- You have appropriate professional liability insurance
- You will NOT make false claims about fat reduction, medical treatment, or detoxification
If conditions are met: Follow ALL manufacturer instructions and safety protocols meticulously. Screen client thoroughly for contraindications—when in doubt, exclude. Obtain informed consent. Prepare treatment area with proper ventilation. Ensure emergency equipment accessible. Inspect device for proper function. Prepare client skin—clean, dry, no products. Explain treatment to client. Position client appropriately. Follow manufacturer guidelines for distance, movement, and duration for specific body area being treated. Maintain constant movement—NEVER keep device stationary. Monitor skin response continuously. Watch client for any signs of adverse reaction (dizziness, difficulty breathing, excessive cold discomfort, cardiovascular symptoms). Treatment duration typically 30-90 seconds per area maximum—do NOT exceed recommendations. Stop immediately if any adverse signs. Allow skin to recover between areas. Do not treat same area multiple times without proper recovery. After treatment, inspect skin carefully. Provide aftercare instructions. Document treatment. Monitor client for delayed adverse effects. Clean and maintain device properly. Store CO₂ tank safely.
Individual Results Vary:
Cold therapy applications may provide temporary effects such as:
- Temporary skin tightening appearance
- Temporary redness/flushing from circulation response
- Temporary feeling of invigoration
- Relaxation from spa treatment experience
However:
- Effects are temporary and superficial
- No evidence this device provides lasting benefits for claimed applications
- Does NOT reduce fat, stimulate significant collagen, or provide medical benefits
- Results are subjective and largely placebo/expectation-based for wellness applications
Most perceived benefits are likely due to:
- Placebo effect (strong for spa treatments)
- Temporary physiological responses to cold
- Professional attention and care
- Relaxation from treatment experience
Rather than any lasting therapeutic effects.
Realistic Expectations:
What This Device Provides:
- Temporary cold therapy application
- Spa/wellness treatment experience
- Professional service offering for facilities
What This Device Does NOT Provide:
- Medical treatment for pain, inflammation, or injuries
- Fat reduction or body contouring
- Significant collagen stimulation
- "Detoxification" (not a real thing)
- Lasting therapeutic benefits
- Medical rehabilitation
- Anything resembling medical cryolipolysis procedures
Regulatory and Legal Considerations:
Professionals using this device must be aware:
- Making medical claims (pain relief, inflammation reduction, injury treatment) without FDA clearance likely violates regulations
- Making false fat reduction claims violates FTC regulations
- Must comply with all local, state, and federal regulations
- Must have appropriate licensing for services offered
- Must maintain proper liability insurance
- Risk of regulatory action, lawsuits if harm occurs
When to Seek Emergency Medical Care:
Call 911 immediately if client experiences:
- Chest pain or pressure
- Difficulty breathing or severe shortness of breath
- Loss of consciousness or altered mental status
- Severe dizziness or confusion
- Signs of stroke (facial drooping, arm weakness, speech difficulty)
- Severe allergic reaction
- Any life-threatening symptoms
For less severe adverse effects, consult appropriate medical professional.
⚕️ IMPORTANT MEDICAL DISCLAIMER The products sold by Strength & Recovery Solutions are intended for general wellness, fitness, and personal use only. None of our products are medical devices, and they are not intended to diagnose, treat, cure, or prevent any disease, medical condition, or injury. We do not make medical or health claims about any products we sell. Any information provided on this website is for general informational purposes only and does not constitute medical advice. Individual experiences and results vary significantly.
Mandatory Health Consultation: You must consult with a qualified healthcare professional before using any fitness equipment, saunas, cold plunge tubs, cryotherapy devices, red light therapy equipment, or other wellness products—especially if you have pre-existing medical conditions, cardiovascular concerns, are pregnant or nursing, or take medications.
Use at Your Own Risk: Users assume full responsibility for proper installation, use, and safety precautions. Follow all manufacturer instructions. We are not responsible for injuries, damages, or adverse effects resulting from use of our products.
"After 6 months with the XMARK Functional Trainer, I can confidently say it's the best investment I've made for my home gym. The cable system is incredibly smooth, and the weight stack provides consistent resistance throughout the full range of motion. Build quality is commercial-grade - feels like equipment you'd find in a premium gym." — Marcus T., Chicago, IL
"I was hesitant about the space it would take up, but the XMARK's compact design fits perfectly in my garage gym. The variety of exercises I can do is endless - from lat pulldowns to functional movements. Assembly was straightforward with clear instructions." — Lisa M., Portland, OR
I’ve had mine for almost a year and couldn’t be happier. It was nearly double the price when I bought it, and I’d still do it again. The tub gets cold fast, feels great, and I love that I can deflate and store it in winter. Support was quick and friendly when I called. Friends and family love using it too. Highly recommend!
--Max B.--

Recovery Solutions
Nationwide Shipping & Handling
Real-time tracking updates.
Exceptional Customer Support
We are available 7 days a week to answer your questions
Secure Payments & Financing Options
SSL-encrypted checkout with secure payment gateways.
Featured collection
⭐⭐⭐⭐⭐
"What truly set Strength & Recovery Solutions apart was the exceptional service I received before I even placed an order. I had a few questions about the equipment and was immediately met with prompt, professional, and knowledgeable support. They took the time to understand my needs and guide me to the right product — no pressure, just genuine care. It felt more like a personalized consultation than a typical sales exchange. A truly elevated experience from the very beginning."
— Paul A., Kansas City, MO
⭐⭐⭐⭐⭐
"From the moment I landed on the Strength & Recovery Solutions website, I knew I was in the right place. The site is beautifully designed, easy to navigate, and feels like a true high-end shopping experience. Every detail — from the product pages to the checkout — was seamless and refined. It’s rare to find a store that combines functionality with such an elevated aesthetic. Absolutely impressed."
— Denise B., Tampa, FL











































